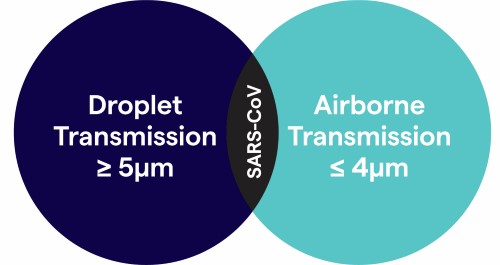
Droplets are considered particles secreted from a patient’s respiratory tract that are no smaller than 5µm in diameter and travel shorter distances (defined as ≤ 3ft) because they cannot stay suspended in air for long periods of time. However, SARS-CoV while still classified as a droplet, is estimated to travel anywhere from 6ft. to 10ft. and can be impacted by environmental factors such as velocity and mechanism by which respiratory droplets are propelled from the source, the density of respiratory secretions, environmental factors such as temperature and humidity, and the ability of the pathogen to maintain infectivity over that distance2 and therefore has the potential to become an aerosol or airborne.
This presents challenges to the assignment of isolation categories because of conflicting information and uncertainty about possible routes of transmission. Although SARS-CoV is transmitted primarily by contact and/or droplet routes, airborne transmission over a limited distance (e.g., within a room), has been suggested, though not proven.3