Overview
Features
The Monarch® System is a HFCWO therapeutic device with revolutionary technology. This vest therapy clears the airways by combining mobility with targeted kinetic energy and airflow to thin and mobilize secretions. Because when patients can move about freely during therapy, they can take control of their therapy—and their lives.
Powered by PODs
The Monarch® System features eight pulmonary oscillating discs (PODs) containing magnets, that are anatomically placed over the upper and lower lobes of the lungs. The PODs oscillate and provide a targeted kinetic energy to the lungs. Airflow is generated to help thin and move mucus from the small airways to the large airways, where it can be coughed out or suctioned.
Mobile
A rechargeable battery powers therapy on the move.
Effective
Study shows that The Monarch® System aids sputum production.1
Connected
App & portal help patients and care teams share therapy and spirometry results.
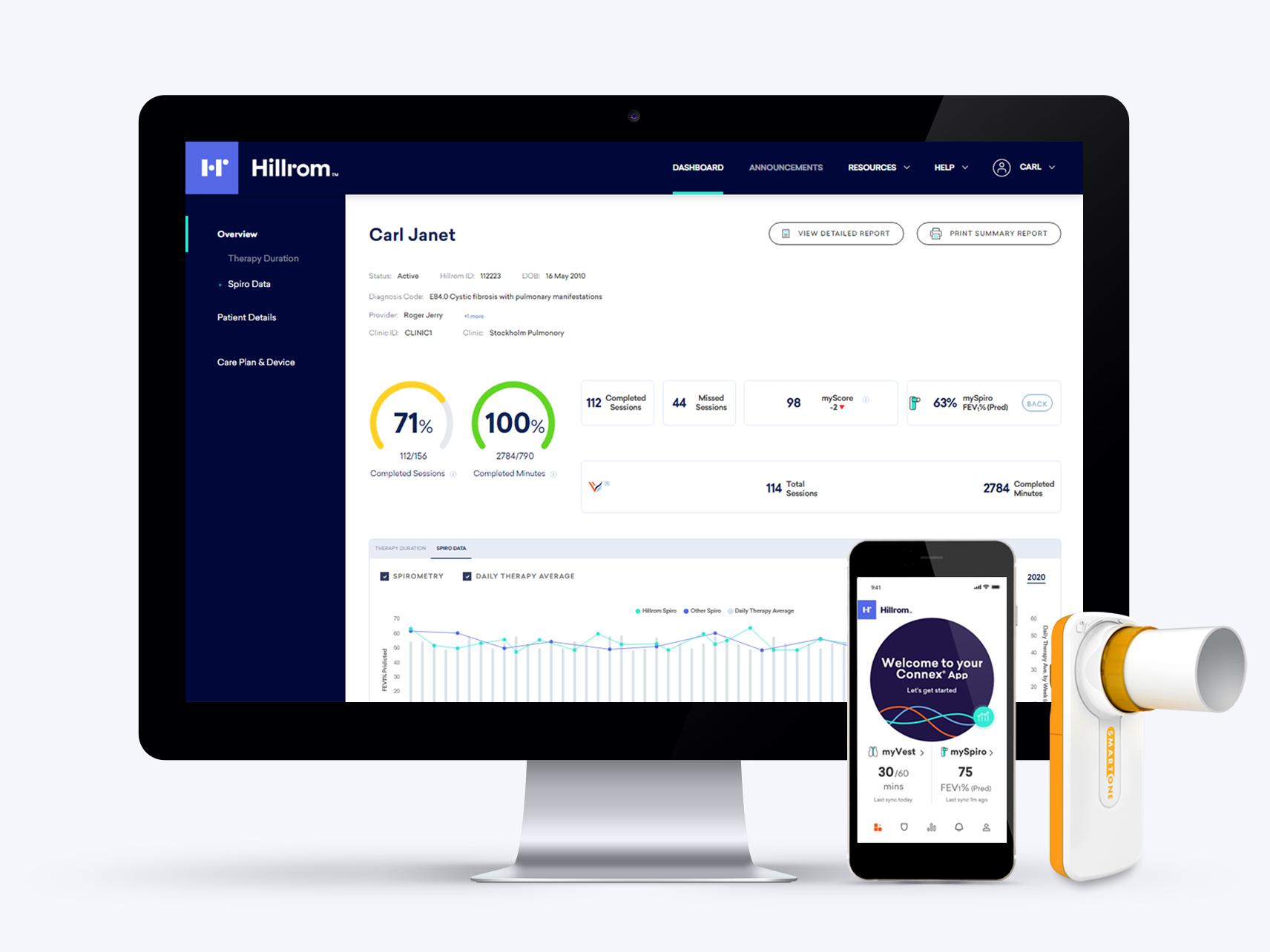
Connection You Can Feel Good About
The Hillrom™ Connex® App and Health Portal help patients and clinicians clearly see day-to-day therapy results, so they can make better decisions together. The app also helps patients keep track of everything from medications and Pulmonary Function Tests (PFTs) to nutrition, exercise and more—rewarding healthy behaviors and empowering them to feel their best. Now using the Smart One® Spirometer, patients can also track lung health at home using the Connex App.
Meet the mind behind the Monarch® System: Marten De Vlieger
Marten De Vlieger and his sister were both born with cystic fibrosis (CF). As they grew up, their mother had to manually administer chest physiotherapy (CPT) to them, to help clear their airways. But Marten envisioned a life with more independence. He created a mobile therapeutic device to provide him with the airway clearance therapy he needed. It freed him to travel, to pursue his love of kite-surfing, to have adventures, and to raise his own family. And when Marten told us that he wanted others to experience the flexibility and control he’d claimed for himself, we gladly partnered with him to develop the Monarch® Airway Clearance System.

Monarch® System 3D Animation Video
See the Monarch® System operation demonstrated in 3D
Technical Specifications
Dig deeper into product attributes to see how we can fit your requirements.
| Weight | 13 lbs. (with battery) |
| Mid-Torso Circumference | Fits 22" to 50" |
| Base Material | EVA foam |
| Shell Material | Polyester |
| Battery | Lithium-ion |
Education & Documentation
Get in the know to get the most value out of your solution.
Product Documentation
-
Videos
keyboard_arrow_downThis 3D animation demonstrates the Monarch® System's features and operation
-
Evidence & Outcomes
keyboard_arrow_downATS BE 3yr Outcomes Data Sheet
Frequently Asked Questions
Expand all-
How do I ensure the Monarch® System fits my patients properly?
keyboard_arrow_downA licensed, professional Hillrom trainer will come to your patient's home to provide a personalized fitting and Monarch® System training. Afterward, your patient may need additional fit adjustments as the Monarch® device's foam conforms to his or her body. If you have any questions about device fit, please call Hillrom Customer Service at 800-426-4224. -
What are the Monarch® System's maintenance requirements?
keyboard_arrow_downAlthough the Monarch® System requires no general maintenance, its cleaning guidelines are listed in its User Manual. -
Are the shells machine-washable and dryable?
keyboard_arrow_downYes. The vest shell can be machine-washed and tumble-dried on low heat. The shoulder pads, backpack cover, and POD covers should be wiped down with an approved cleaner. Refer to the Monarch® System's User Manual for full cleaning guidelines. -
How does the Monarch® System provide therapy to the entire lung when the PODs are only touching the torso in eight places?
keyboard_arrow_downExcellent question. The Monarch® System employs a clearance therapy known as High Frequency Chest Wall Oscillation (HFCWO) to thin and mobilize secretions from the airways and help improve respiratory health. Its Pulmonary Oscillating Discs (PODs) deliver targeted kinetic energy to both lungs' upper and lower lobes, front and back. This helps to thin mucus and increase airflow throughout the lungs. That stronger airflow can get behind secretions, lift them away from airway surfaces, and move them along from the small airways to the large airways, from which they can be more easily coughed or suctioned out. -
How does the Monarch® System's therapy differ from that of the Vest® System?
keyboard_arrow_downBoth provide HFCWO therapy to clear the airways. Whereas the Vest® System uses an airbladder system to deliver airflow-generating energy over the entire chest wall, the Monarch® System uses its magnet-containing PODs deliver energy to specific regions of the lungs' upper and lower lobes, therefore wearing a Monarch® garment may feel different from wearing a Vest® garment. The Monarch® System's targeted kinetic energy likewise thins mucus and generates airflow to mobilize secretions. -
Do health insurance companies typically cover the Monarch® System?
keyboard_arrow_downContact your (or your patient's) insurance company to determine coverage for High Frequency Chest Wall Oscillation (HFCWO) devices. The Monarch® System is intended for patients who are 15+ years old. -
How often does Hillrom contact Monarch® patients?
keyboard_arrow_downA member of the Hillrom Respiratory Health’s clinical staff will periodically call the patient to discuss how the system is working for him or her and to answer any questions. Insurance providers may request this data to ensure that the device is being used. -
Can I travel on an airplane with the Monarch® System?
keyboard_arrow_downWhen traveling with the Monarch® System, it's recommended that patients review the latest medical device regulations on the U.S. Transportation Security Administration's website (www.tsa.gov). The Monarch® System has a lithium-ion battery and current TSA guidelines allow a lithium-ion battery to be included only in carry-on luggage (it may not be included in checked luggage). When a bag is checked at the gate or at plane-side, the Monarch® System's battery must be removed and kept with the passenger in the aircraft cabin. The travel agent or airline should be able to answer questions regarding the requirements for traveling with medical devices. Patients might also consider carrying a letter from their physician, stating their medical condition(s) and medical need for the device.











